The efficient interconversion between O2 and water is central to many energy systems, from respiration and photosynthesis to emerging energy technologies. The oxygen reduction reaction (ORR), O2 + 4e– + 4H+ -> 2H2O, is the cathode reaction in fuel cells. The production of fuels from solar energy requires electrons and these electrons typically come from the opposite process, the oxygen evolution reaction (OER), 2H2O -> O2 + 4e– + 4H+. New approaches and better catalysts and electrocatalysts are needed for these reactions to replace precious metals with earth-abundant electrocatalysts and to achieve higher efficiencies (high rates, high selectivity, long lifetimes, and low overpotentials).
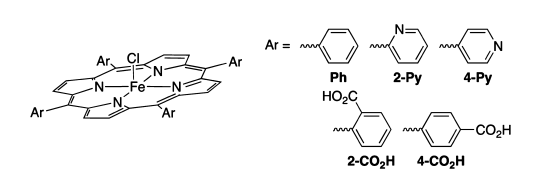
(i) We have developed new iron-porphyrin ORR electrocatalysts that incorporate potential proton relays. These potential relays are oriented towards the iron, so that they could perhaps deliver proton(s) to a ligand derived from O2. Such relays do appear to enhance the selectivity of O2 reduction, favoring the 4e–/4H+ reduction to H2O rather than the 2e–/2H+ reduction to H2O2. However, ongoing studies are showing that the roles of the relays are complex.

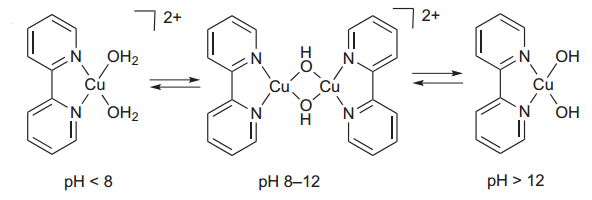
(ii) We reported the first molecular copper OER electrocatalyst, a simple complex of copper(II) and a bipyridine ligand, which speciates depending on the solution pH. Despite this simplicity, or perhaps because of it, understanding the mechanism and extending to heterogenized systems is challenging. However, the observation of electrocatalytic water oxidation is exciting and proceeds at fast rates, albeit at somewhat high overpotentials.