Transition metal complexes can often serve as valuable models for active sites in metalloproteins and other biochemical processes. Our focus is on modeling and understanding the reactivity of these sites, especially the PCET reactivity, more than the structure or spectroscopic properties of the metal cofactor. PCET processes in biological systems are often difficult to study in detail because the surrounding water provides a sea of protons. In model systems that utilize aprotic organic solvents, it is much easier to identify the key proton that does, or does not, accompany an electron transfer process. Ideally, these biomimetic systems both provide new insight into biochemical reactivity and build fundamental understanding of PCET, as described in Hydrogen atom transfer and Multiple-site proton-coupled electron transfer areas.
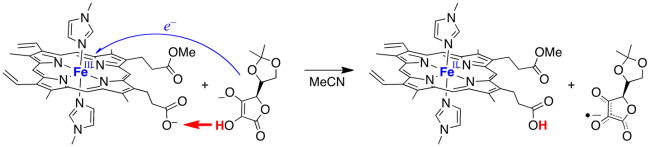
In one example, we prepared an iron complex of protoporphyrin IX, the ubiquitous heme in hemoglobin, cytochrome P450s, and many other enzymes. We bound the iron with two ligands and prepared the monomethyl ester, so that only one heme propionate was available to accept a proton. In the oxidized iron(III) form, this complex oxidizes ascorbate with transfer of the electron to the iron and the proton to the propionate (by MS-CPET). This complex is therefore a model for the reactivity of the related high oxidation state forms of ascorbate peroxidase.
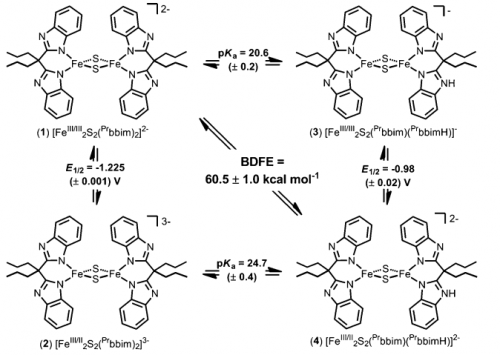
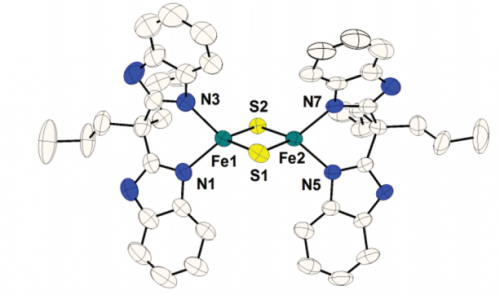
In another study, we demonstrated PCET reactivity of a synthetic two-iron, two-sulfur cluster with imidazole/imidazolate ligands. Their quinone/hydroquinone reactivity is a model for the chemistry of the Rieske cluster in the mitochondrial bc1 complex, and for the chemistry of other related iron-sulfur clusters.