Redox reactions across the interface between a solid and a solution are ubiquitous in the environment and in many technologies. These are usually discussed as pure electron transfer processes, but our laboratory has recently shown that these reactions can be proton-coupled electron transfers. It is our hypothesis that protons are often key components of redox chemistry of these nanocrystals, and that the coupling of the electrons with protons is a common and important component of many processes, although currently underappreciated.
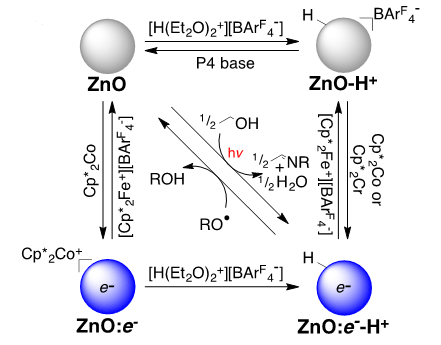
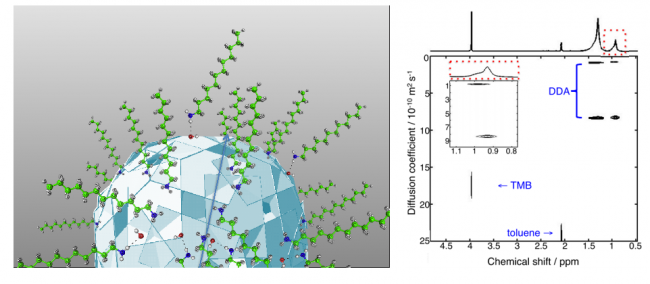
We are working mostly with colloidal metal oxide nanocrystals, including ZnO, TiO2 and CeO2, as well as molybdenum nitride (Mo2N). These colloidal materials can be studied using many of the techniques of molecular chemistry, such as titrations and optical, NMR, and EPR spectroscopies. We are also using materials characterization techniques such as x-ray diffraction and electron microscopy. We are exploring inner-sphere reactions of these materials, redox reactions that involve bond making and bond breaking, for instance O–H bonds at the surface of a metal oxide. We are examining the stoichiometries and mechanisms of these novel reactions. What we learn from these fundamental studies should be relevant to a wide range of applications.