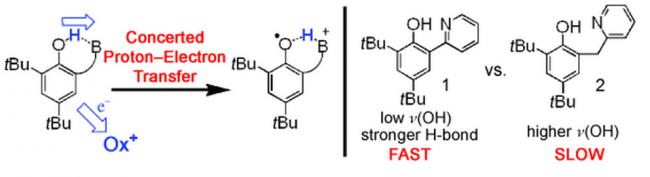
Our laboratory has developed systems to examine PCET oxidations of phenols and related molecules in detail. We are constructing phenols with intramolecular hydrogen bonds, so that upon oxidation of the phenol portion the proton moves in a very controlled way. In the example below, the phenol transfers an electron to an oxidant A+ concerted with proton transfer to a pyridine. The oxidant could be an organic or inorganic reagent, or a photochemical excited state. The advantage of photoinduced MS-CPET is that very fast processes can be studied. These molecules are in some ways models for the archetypal example of MS-CPET is the redox active tyrosine Z in Photosystem II. Our systems have provided insights into the dependence of MS-CPET reactions on driving force (the sum of the electron transfer and proton transfer energetics), on the Marcus intrinsic barriers (rearrangements that occur in concert with MS-CPET), and the nature of the hydrogen bond. As shown in the figure below, stronger H-bonds – as indicated by lower ν(OH) – exhibit faster CPET rates.