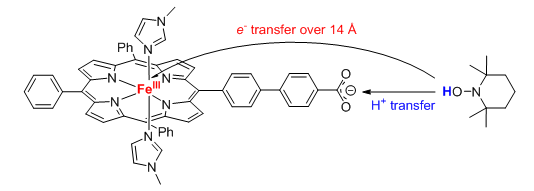
Many PCET reactions occur by transfer of e– and H+ to or from different locations, and therefore do not resemble hydrogen atom transfers. When the e– and H+ transfer in the same kinetic step, these are called multiple site, concerted proton-electron transfer reactions (MS-CPET). Such reactions are increasingly recognized in biological systems, and likely play an important role in many catalytic and electrocatalytic PCET processes. We are interested in PCET reagents in which the e– and H+ transfer to or from very separated sites. In the example below with an iron porphyrin (heme), proton transfer to the carboxylate is concerted with quite long range electron transfer (14 Å). Other examples are given in the Biomimetic reactivity of transition metal complexes section.
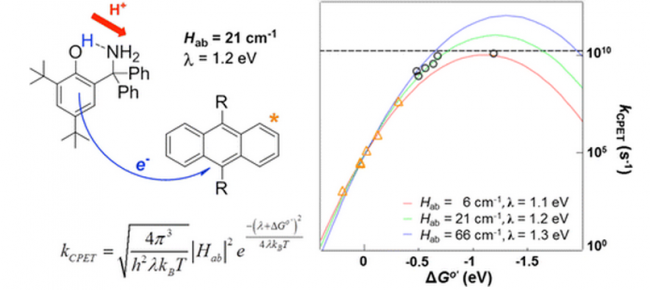
We are also preparing and examining organic molecules designed to react by MS-CPET, as described in the Probing PCET with organic molecules section. These studies are revealing the effects of distance – the distances over which the electron and proton travel, and the distances between the e– and H+ in the initial and final states. We are also probing the role(s) of the interaction or coupling between the electron and proton, including the basic questions of what is meant by this coupling and how can we measure it. The results are being analyzed with Marcus Theory and sometimes more sophisticated theories of PCET.