HAT: e– and H+ move together
Our laboratory has developed a detailed and often predictive understanding of hydrogen atom transfer reactions of metal-containing active sites. These are central to a variety of metalloenzymes, such as cytochrome P450s and non-heme Fe and Cu oxidases. Such reactions are one type of PCET reaction, in which e– and H+ transfer from one reagent to another: AH + B <-> A + BH. We have shown that a wide variety of metal active sites can react by hydrogen-atom transfer, including Fe, Mn, Co, Cu, Cr, Ru, and Os complexes, iron porphyrin compounds and iron-sulfur clusters. We have developed a model based on Marcus Theory that rationalizes and can quantitatively predict this reactivity. The key parameters in this model are the driving force (∆G°) for H-atom transfer – the strengths of the bonds being cleaved and formed – and the Marcus intrinsic barrier. As shown in the figure below, the Marcus cross relation holds for a variety of metal complexes over a large range of driving forces and rates.
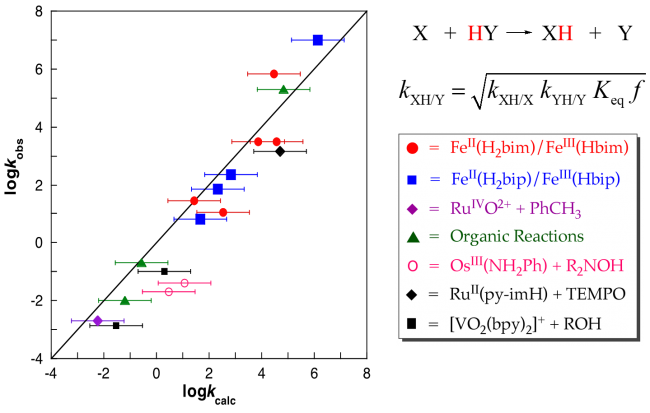
This model also encompasses organic H-atom transfer reactivity, which occurs in significant processes such as oxidative stress and antioxidant behavior, as well as in many enzymes. Our approach therefore shows the commonalities between transition metal and organic H-atom transfer processes. We are very pleased that this fundamental understanding of H-atom transfer reactions is being increasingly used in studies of enzymes and model systems.
